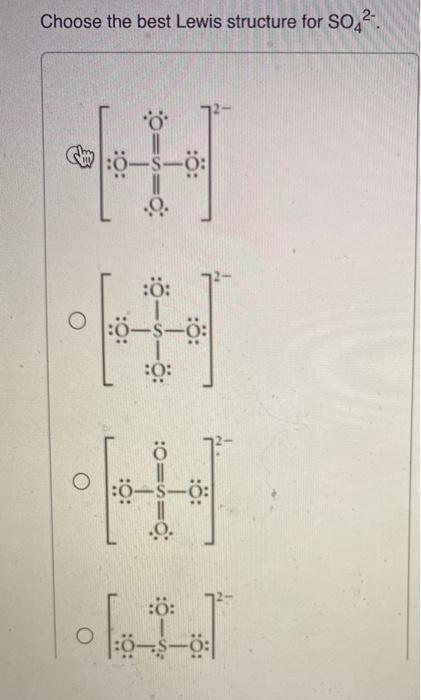
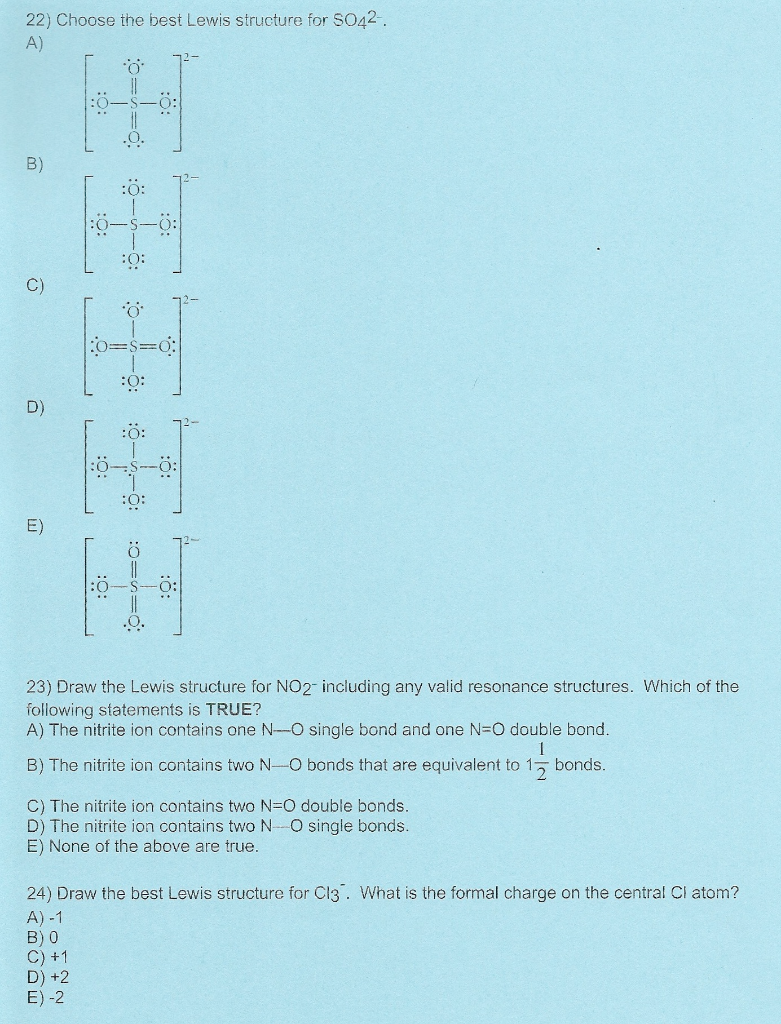
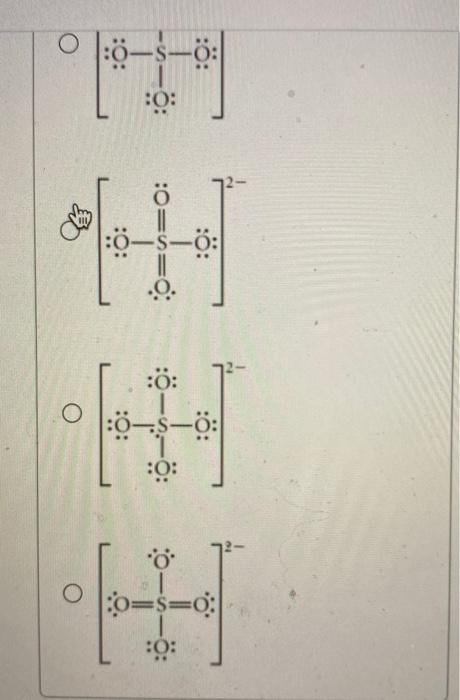
Choose The Best Lewis Structure For So42-.
Choose the best lewis structure for so42-.. Use the data below to construct a born haber cycle to determine the lattice energy of CaO. Choose The Best Lewis Structure For SeO42-. When youre done put brackets along with 2- around the Lewis structure for SO 42- to show that it is an ion with a negative two charge.
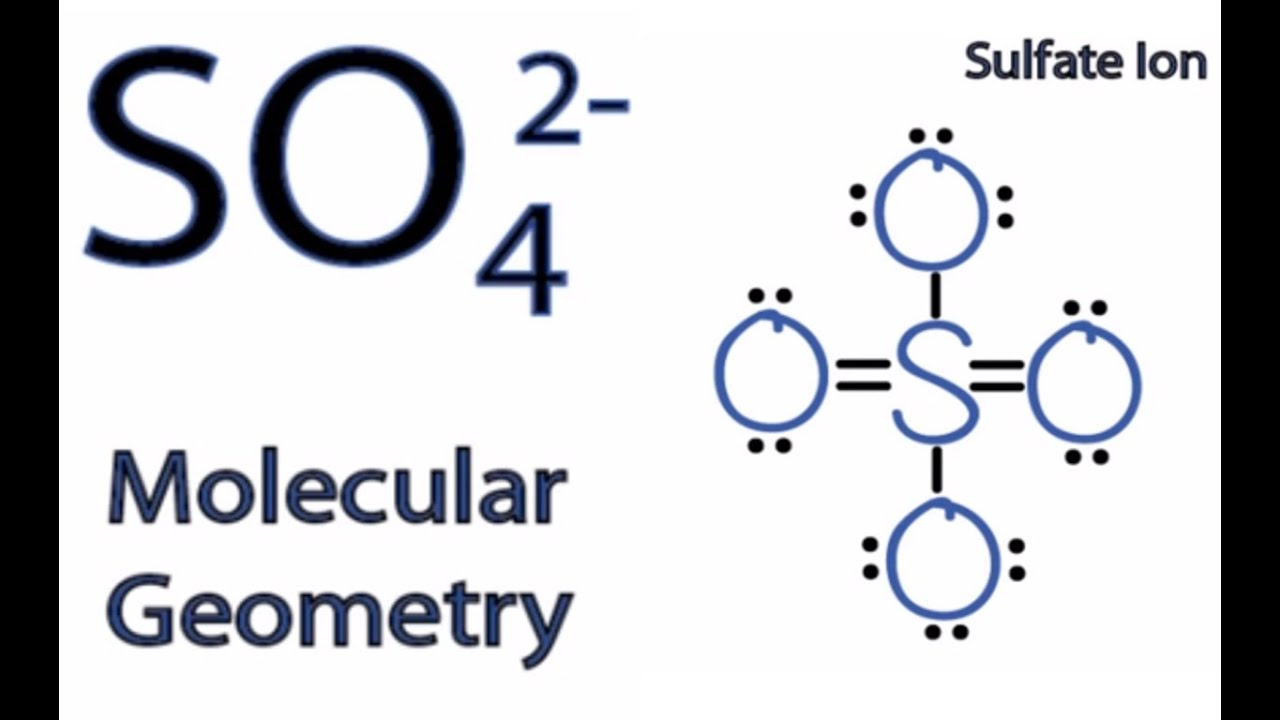
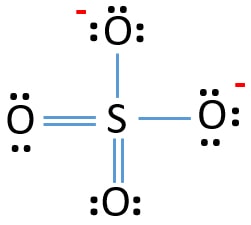
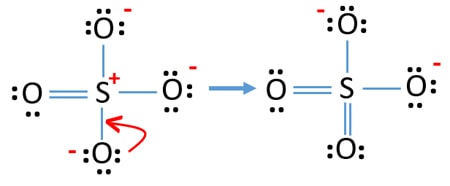
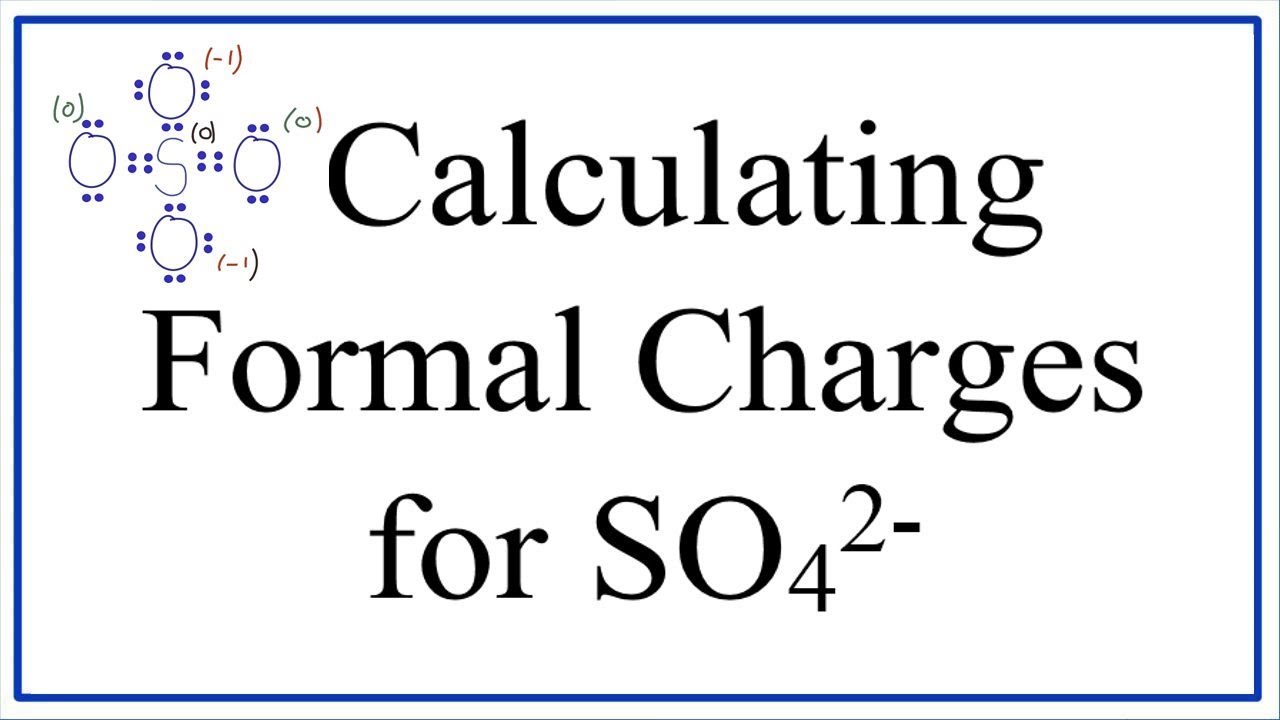
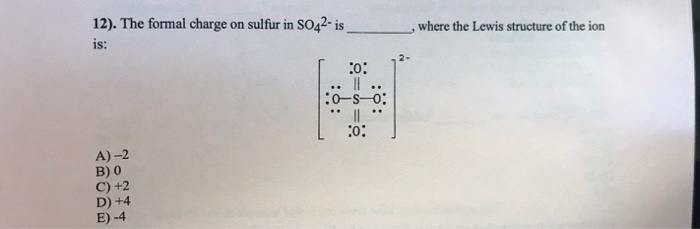
In SO 42- lewis structure there are four oxygen atoms around the sulfur atom. 017 H 41 ö. D Draw the Lewis structure for the free radical NO2 and determine the formal charge on the N.
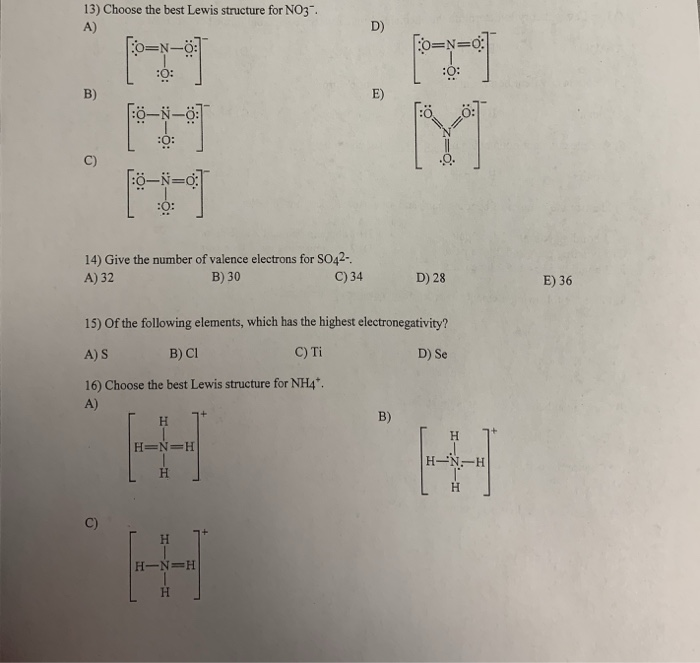
Write the name for SnSO42. Choose the best Lewis structure for ICl5. View Test Prep - Practice Final from CHEM 1465 at University of Texas Arlington.
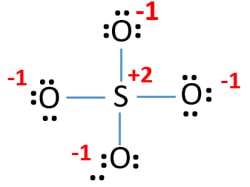
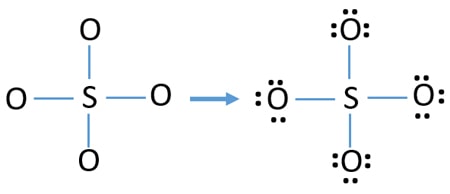
Two of the oxygens are single-bonded and two are double-bonded. Choose the best Lewis structure for SO4 2-Draw the best Lewis structure for Cl3-. There are 32 valence electrons available for the Lewis structure for SO 42-.
I quickly take you through how to draw the Lewis Structure of SO4 2- Sulfate Ion. Drawing the Lewis Structure for SO 42- Sulfate Ion Sulfates salts with the SO 42- are frequently used in industry and biologically. I also go over hybridization shape and bond angles.
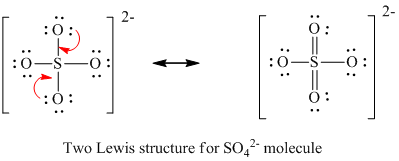
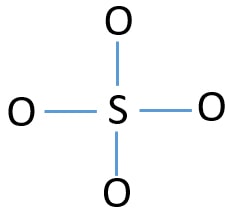
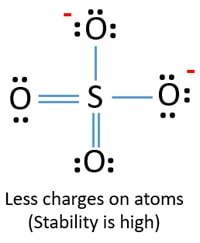
Youll need to place a double bond on two of the Oxygen atoms in order to have the best Lewis structure. Cl - Cl - Cl -. Lewis structure of SO 42- There are two SO bonds and two S-O bonds in sulfate ion lewis structure.
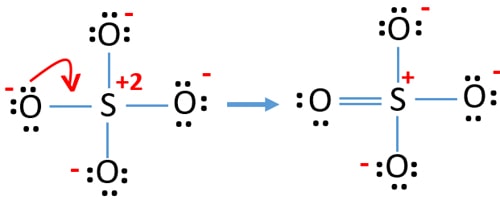
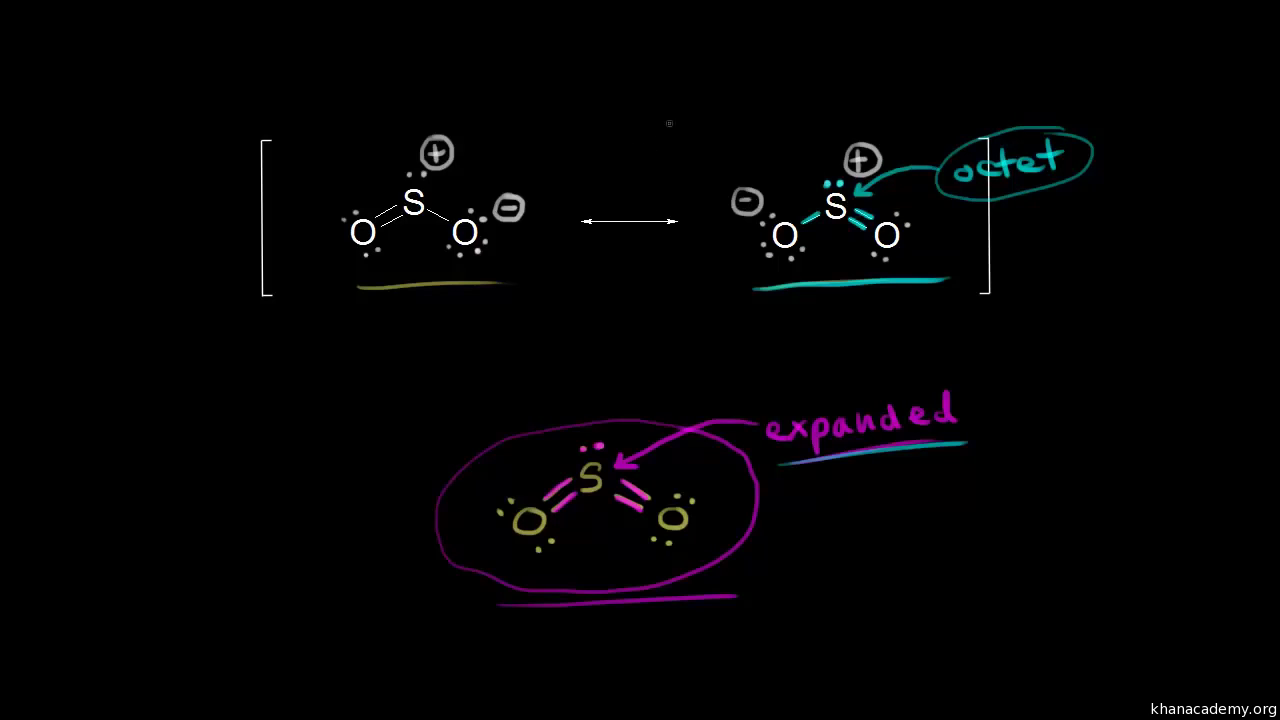
23 Draw The Lewis Structure For NO2 Including Any Valid Resonance Structures. The reason is FORMAL CHARGE and the fact tha.
Cl - Cl - Cl -.
Choose the best lewis structure for XeI2. In lewis structure there should. View Test Prep - Practice Final from CHEM 1465 at University of Texas Arlington. In SO 42- lewis structure there are four oxygen atoms around the sulfur atom. Two of the oxygens are single-bonded and two are double-bonded. I- 6 dots Xe-6 dots I-6 dots single bonds. Choose the best Lewis structure for SO42-. Drawing the lewis structure for hcn. There are 32 valence electrons available for the Lewis structure for SO 42-.
I also go over hybridization shape and bond angles. Remember that Sn forms several ions. Lewis structure of SO 42- There are two SO bonds and two S-O bonds in sulfate ion lewis structure. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo toothpaste etc. I quickly take you through how to draw the Lewis Structure of SO4 2- Sulfate Ion. Ions practice you can also practice Lewis Dot Structures. SO 42- has a total of 32 valence electrons remember that -2 charge counts as two valence electrons.































Post a Comment for "Choose The Best Lewis Structure For So42-."